- Galvanizing TRIP and 3rd Generation AHSS Grades
- New Alloy Product Development and Characterization: Complex Phase Steels
Galvanizing TRIP and 3rd Generation AHSS Grades
The background for this section comes from Citation B-93.
Galvanizing of TRIP and 3rd Generation AHSS grades is particularly challenging.
Manganese (Mn), aluminum (Al), and silicon (Si) are used in TRIP steel for strengthening, promoting austenite stability (Mn) and preventing cementite precipitation which indirectly enhances retained austenite stability (Si and Al).
The typical heat treatment used to produce the targeted TRIP microstructure in uncoated steels is not compatible with the hot dip galvanizing thermal cycle, and results in a loss of the desired properties.
Heat treatment of uncoated TRIP steels usually starts with intercritical annealing (IA) typically at temperatures in the range of 750 °C to 900 °C, depending on the alloy and the targeted properties. This is followed by holding at an isothermal bainitic transformation (IBT) of 360 °C to 410 °C to produce the best combinations of the proper microstructure, tensile strength, and uniform elongation. However, this heat treatment cycle is not compatible with continuous galvanizing lines, where the zinc bath is typically held at around 465 °C and the incoming steel strip enters at a slightly higher temperature.B-93
An IBT holding temperature at the higher temperatures typical of galvanizing undesirably impacts the microstructure developed at the lower IBT. The higher temperatures promote carbide precipitation, resulting in the final microstructure having a lower volume fraction of lower carbon retained austenite – neither of which are associated with good TRIP properties. This lower C content retained austenite will have lower stability, resulting in a rapid retained austenite to martensite transformation and mechanical properties characteristic of dual phase steels.B-93
In addition, the speed at which coils move through continuous galvanizing lines dictates how long the steel spends in each step, including the galvanizing pot. The time spent at the galvanizing pot temperature is shorter than necessary to produce the desired properties, at least with the chemistry used for uncoated TRIP steels. TRIP steels with higher aluminum levels are potential alternatives for galvanized versions, since they may allow for targeted microstructure development at the shorter times associated with strip travel through the galvanizing pot, and result in adequate carbon enrichment in the retained austenite and as such providing the desired stability to this phase. Furthermore, partially or completely replacing SI with Al has been shown to improve reactive wetting in galvanized TRIP steels. Aluminum additions also may have the benefit of suppressing the depth and number of internal oxides, in particular at grain boundaries during coiling after hot rolling.L-76
Furthermore, the primary alloying elements used in TRIP steel – Mn, Al, and Si – undergo selective oxidation during the annealing portion of continuous galvanizing lines. Without proper control of the dew point and the oxygen potential of the annealing atmosphere, thick oxides cover the surface and result in poor reactive wetting and unacceptable bare spot defects in the Zn coating.B-97 This applies to both hot dip galvanizedC-47 and hot dip galvannealed zinc coatings.C-48
The presence of chromium (Cr) affects oxide layer formation, with the impact also being a function of the dew point and the oxygen potential of the annealing atmosphere. Formation of a Cr2O3 oxide layer, which blocks penetration of additional oxygen to the steel beneath it, is the same mechanism that makes stainless steels “stain less” and improves corrosion resistance.Z-22
Poor wettability is shown in Figure 1, using an example from Citation D-49.
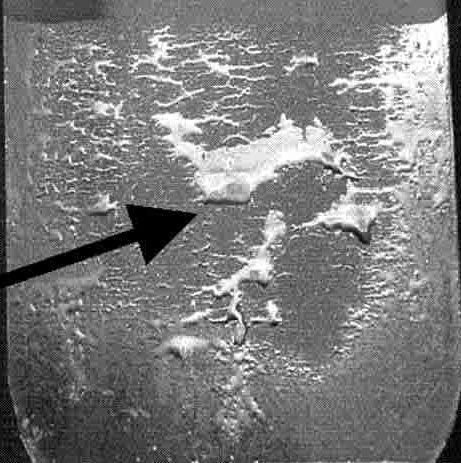
Figure 1: Poor wettability after galvanizing. SiO2 covers the surface in the bare areas, while the sparse areas covered with zinc correspond to reaction areas with an interfacial Fe2Al5 layer in between the zinc and steel. Modified from Citation D-49.
The dew point below which wettability problems can start is a function of the alloy chemistry and other conditions in the annealing furnace. Figure 2 highlights the transition from a non-wettable to wettable surface as the dew point increases from −35 °C to −26 °C.

Figure 2: Wettability improves as the dew point increases from −35 °C to −26 °C.D-49
Other Strategies to Improve Galvanizability
As reported in Citation G-57, methods used to improve the galvanizability of TRIP steels include:
- Use of a high dew point atmosphere, resulting in the internal selective oxidation of Si and Mn to SiO2 and xMnO.SiO2, respectively;
- Pre-deposition of a thin layer of Fe, Ni or Cu to prevent selective oxidation altogether.
- Pre-oxidizing the steel surface to form isolated, rather than continuous, oxide particles which are embedded in a thicker Fe-oxide surface layer. This layer is subsequently reduced to metallic Fe in a reduction step.
These approaches are summarized in Figure 3.
Figure 3: Potential methods to promote zinc wettability and avoid bare spots during hot dip galvanizing of TRIP steels.G-57
TRIP steels contain several elements with strong tendencies to oxidize, namely manganese (Mn), and silicon (Si), in addition to the iron (Fe) in the alloy. Carbon plays no direct role in surface oxidation but can be lost by decarburization at high dew points where carbon combines with oxygen to form either CO or CO2.
By using a slightly oxidizing annealing atmosphere and a slightly higher dew point than typical annealing atmospheres (approaching 0°C, instead of −40 °C),B-94, B-95, B-96, N-34 it is possible to preferentially oxidize Fe to form a thin surface FeO layer before Mn and Si can oxidize externally. Manganese and silicon are prevented from reaching the surface, and any Mn or Si oxides form beneath the iron oxide layer or at grain boundaries rather than at the top surface.
Next, the steel passes through a strongly reducing atmosphere at a lower dew point (−20 °C or preferably lower)J-32, G-57 and greater hydrogen concentration.
The iron oxide surface layer is reduced back to metallic Fe by hydrogen, producing iron and water vapor. The internal oxides (MnO, SiO₂) remain beneath the surface where they do not impact zinc wettability.
The steel surface after this oxidation-reduction process is primarily iron, offering substantially improved zinc wettability and adhesion with a reduced risk of bare spot defects in the galvanized zinc coating.
Deployment of this oxidation-reduction method relies on accurate control of atmospheric conditions at the oxidation and reduction chambers.
New Alloy Product Development and Characterization: Complex Phase Steels
In addition to being able to produce a new grade, it is important to consider the applications where it might be used, and the characteristics it must have. In the past, it might have been sufficient to design only for tensile strength and possibly ductility as measured by total elongation in a tensile test. But the industry is demanding much more than that from today’s advanced steels.
An example of some of the efforts involved in new alloy characterization are found in Citation R-30, where advanced testing involved with commercializing a hot rolled High Strength Low Alloy steel and a hot rolled Complex Phase steel are highlighted.
Metallurgically, the desire was to move away from single-phase ferritic or bainitic steels that rely on micron-sized second-phase constituents for higher strength, but also produce damage and voids upon shearing, to an approach that derives strength from nanometer-sized precipitates that do not promote substantial void formation during shearing.
The typical evaluation for cut edge stretchability is the hole expansion test, standardized under ISO 16630. This test is used to assess stretch-flangability and to predict edge-cracking during manufacturing. The test evaluates the steel’s resistance to through-thickness crack propagation in a shear-affected and work-hardened zone from
punching.
However, the stress and strain conditions imposed on edges during high volume manufacturing may be more complex than those found in the hole expansion test.
The study in Citation R-30 used a deep-draw-cup (DDC) test to screen multiple alloying approaches of the targeted grades for crack susceptibility and fracture toughness. This outcome was used further to study the relationship between hole expansion and toughness, and the associated contributions of microstructural features like grain size, second-phase fraction, and texture. Additional information about the specifics of the tests and results are found elsewhere.R-31
Alloy composition impacts strength and formability, but also influences corrosion resistance, which is an important consideration in hot rolled steels for frame and chassis applications.
For the alloying, different balances of niobium, vanadium, and titanium, and molybdenum were used to influence austenite recrystallization and r-values in the HSLA grades with the target of suppressing the formation of carbon-rich second-phase constituents, other than nanometer-sized carbide and carbonitride precipitates. In the complex phase steels, boron and titanium were used to promote a titanium carbide-strengthened single-phase bainitic microstructure. In these, the C content was varied to promote differences in carbon-rich second-phase fractions in the bainitic microstructure (martensite and cementite).
The mill processing route impacts properties. After heating to 1240 °C, lab samples were rolled down to 3.5 mm. Finish-rolling temperatures (FRT) of 980 and 890 °C were used to study the effect of finish rolling on planar anisotropy and grain size. After hot rolling, the plates directly transferred to a run-out-table (ROT) and cooled with a mixture of water and pressurized air before being placed in a furnace to replicate coil cooling. For the HSLA steels, the exit ROT and furnace temperatures were 580 and 630 °C. For the complex phase steels, the exit ROT and furnace temperatures were 400 and 450 °C. The effect of a change in microstructure (e.g., grain size, second-phase constituents) on HEC and toughness was investigated by varying the coiling temperature (CT).
Microstructural characterization was done with Scanning Electron Microscopy (SEM) and Electron Backscatter Diffraction (EBSD), in addition to X-Ray Diffraction (XRD) for texture analysis.
Each condition was evaluated for tensile properties, as well as DDC testing for susceptibility to crack formation and the energy required for crack growth, Hole Expansion testing, and Charpy V-notch (CVN) impact testing at -60, -40, -20, 0, and +20 °C to determine the absorbed energy and ductile-to-brittle transition temperature.
Even though the hole expansion values between the HSLA approach and the CP steels were similar, the bainitic CP steels exhibit substantially lower crack susceptibility and increased toughness than ferritic HSLA steels in the deep-draw cup (DDC) test. The DDC test was capable of detecting differences in crack susceptibility and work of fracture for different steel types under test conditions that simulate industrial manufacturing conditions.
Concurrent with these microstructural and mechanical tests, samples were evaluated for corrosion resistance using SAE J2334, VDA 233-102, and VDA 621-415.
No one alloy chemistry and processing route resulted in a single answer having the best performance in all of the characteristics evaluated, but this wide scope of testing provided the data that the steelmaker used in commercializing a new grade, HR660Y760T-CP.
Instead of the conventional focus on stretch-flangability and hole expansion, the approach was widened to additionally include fracture toughness to assess crack susceptibility under conditions that take into account secondary cold work embrittlement and replicate component manufacturing through successive forming operations with alternating compressive and tensile stresses. The detailed investigation also provided guidance on which alloying elements could be reduced to result in more cost-effective production without compromising performance.